The Tyndall Effect is the effect of light scattering in many directions in colloidal dispersion, while showing no light in a true solution. This effect is used to determine whether a mixture is a true solution or a colloid. “To be classified colloidal, a material must have one or more of its dimensions (length, width, or thickness) in the approximate range of 1-1000 nm.”
Because a colloidal solution or substance (like fog) is made up of scattered particles (like dust and water in air), light cannot travel straight through. Rather, it collides with these micro-particles and scatters causing the effect of a visible light beam. This effect was observed and described by John Tyndall as the Tyndall Effect.
The Tyndall effect is an easy way of determining whether a mixture is colloidal or not. When light is shined through a true solution, the light passes cleanly through the solution, however when light is passed through a colloidal solution, the substance in the dispersed phases scatters the light in all directions, making it readily seen.
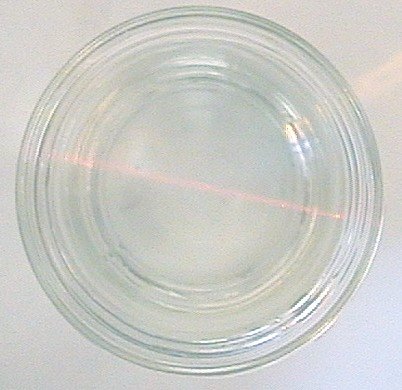
This picture was taken from above and shows the Tyndall effect in subdued light. The laser was directed from right to left. If one were to look at the vessel from in front at eye level, the Tyndall effect would be almost invisible. It’s hard to see but the red beam is striking the silver particles in the water and what is seen is the light reflecting off those particles. A strong Tyndall effect would indicate large particles. This picture was taken of our colloidal silver made to 20 PPM strength. By using constant current and also stirring the CS during production, we are able to make a quality product. This is what one should see when looking for Tyndall
A laser will allow one to see the Tyndall effect very easily. Just direct it through the colloidal dispersion and the beam will strike the particles and the light will be reflected off those particles. Large particles will be seen as what is known as “sparklers”.

Leave A Comment
You must be logged in to post a comment.